
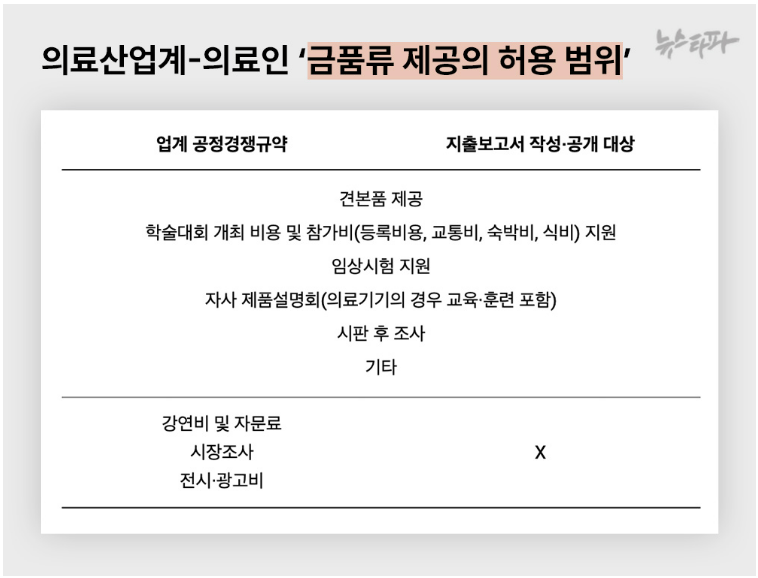
1. Information related to the management or business secrets of a corporation, an organization or individual that is recognized as likely to significantly harm the legitimate interests, if disclosed2. Other information recognized by the Minister of Health and Welfare-Article 44, paragraph 4, etc. of the Ordinance on Partial Amendment in the Implementation Regulations of the Pharmaceutical Affairs Act

| Design | Lee Do-hyeon |
| Web Publishing | Sim In-bo |
뉴스타파는 권력과 자본의 간섭을 받지 않고 진실만을 보도하기 위해, 광고나 협찬 없이 오직 후원회원들의 회비로만 제작됩니다. 월 1만원 후원으로 더 나은 세상을 만들어주세요.