
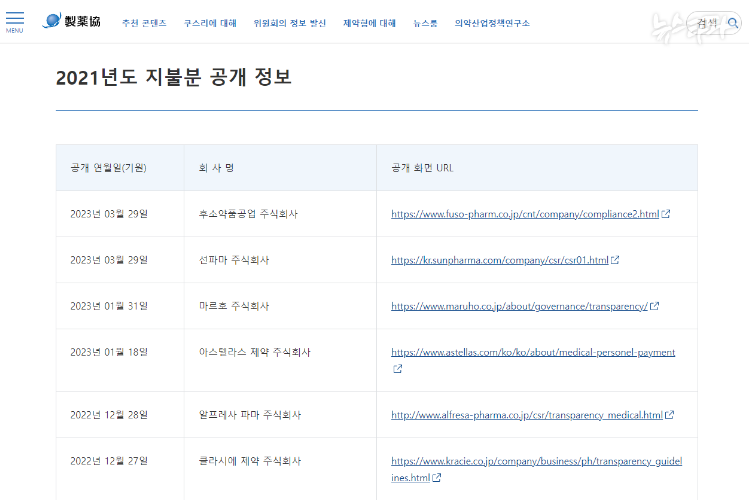
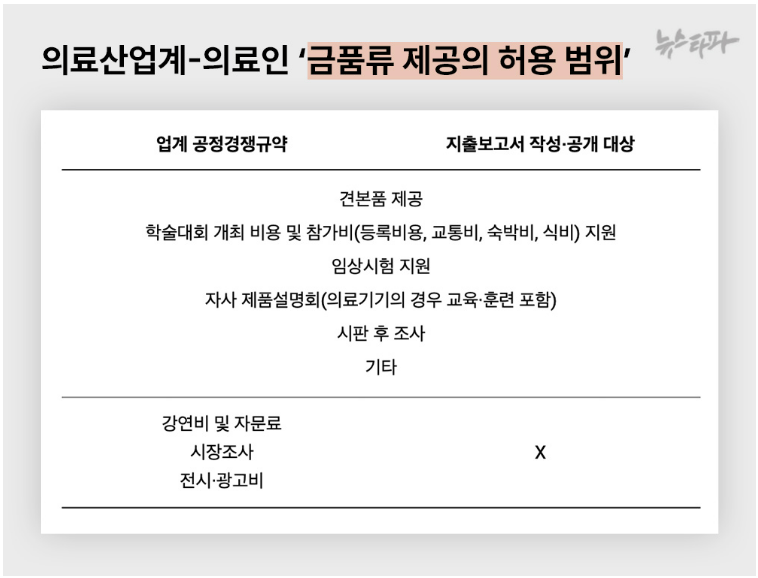
1. Information related to the management or business secrets of a corporation, an organization or individual that is recognized as likely to significantly harm the legitimate interests, if disclosed2. Other information recognized by the Minister of Health and Welfare-Article 44, paragraph 4, etc. of the Ordinance on Partial Amendment in the Implementation Regulations of the Pharmaceutical Affairs Act

| Design | Lee Do-hyeon |
| Web Publishing | Sim In-bo |
KCIJ-Newstapa does not accept any advertisement or commercial sponsorship. Individual citizen's voluntary support sustains Korea’s only independent investigative newsroom. You can join our 'Defenders of the Truth' now.